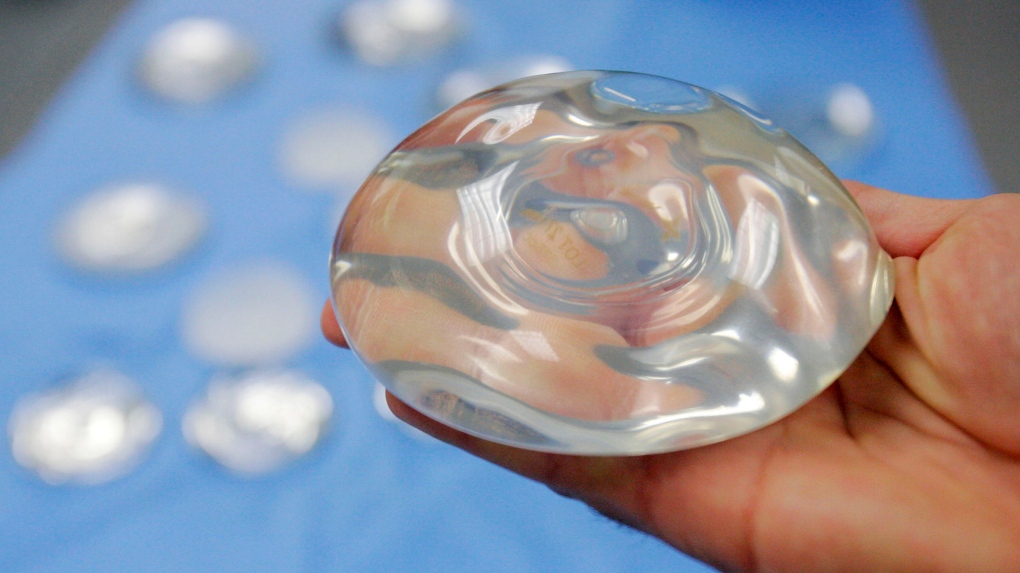
Patients and doctors in Canada are welcoming the possibility of a national registry for breast implants, which is long overdue and crucial for public safety. The House of Commons Standing Committee on Health recently heard submissions for a feasibility study into creating a nationwide registry, which follows years of investigations and advocacy by women who have been harmed by the devices. Advocates argue that a national registry containing details of every implant performed in Canada is necessary for patients and researchers to track illnesses, injuries, and other information. Dr. Peter Lennox, a clinical professor of plastic surgery at the University of British Columbia, said that he had reached out to Health Canada in 2017 to recommend creating a registry, but nothing progressed.
Nancy Pratt, president of the Breast Implant Failure and Illness Society, stated that calls for a registry date back to the 1990s, and that no one has been willing to take responsibility for tracking implants, which is alarming since foreign objects are placed in the human body with no way for patients and their doctors to track any problems. Pratt argued that any registry should not just be limited to implants, but should also include other materials like mesh and clips that may be placed inside the body during implant surgery. Health Canada spokesperson Mark Johnson wrote in an email that a breast implant registry is a complex endeavor that would require participation and coordination from multiple levels of government, and that any next steps would heavily depend on its purpose, structure, and design.
Dr. Jan Willem Cohen Tervaert, a professor of medicine at the University of Alberta, said that the medical device industry, and especially the breast implant medical device industry, needs more supervising since authorities are failing to do so. He pointed to the example of Poly Implant Prothèse, a French company that was revealed in 2010 to be selling implants made from industrial-grade silicone rather than the mandatory medical-grade product. The company’s founder ended up in prison for fraud as a result. Cohen Tervaert stated that the Netherlands has a very robust breast implant registry, which is now covered by patients, who pay the equivalent of about $40 when they have surgery. The registry operates on an opt-out basis, meaning it is mandatory unless patients choose not to have their information included in the database.